
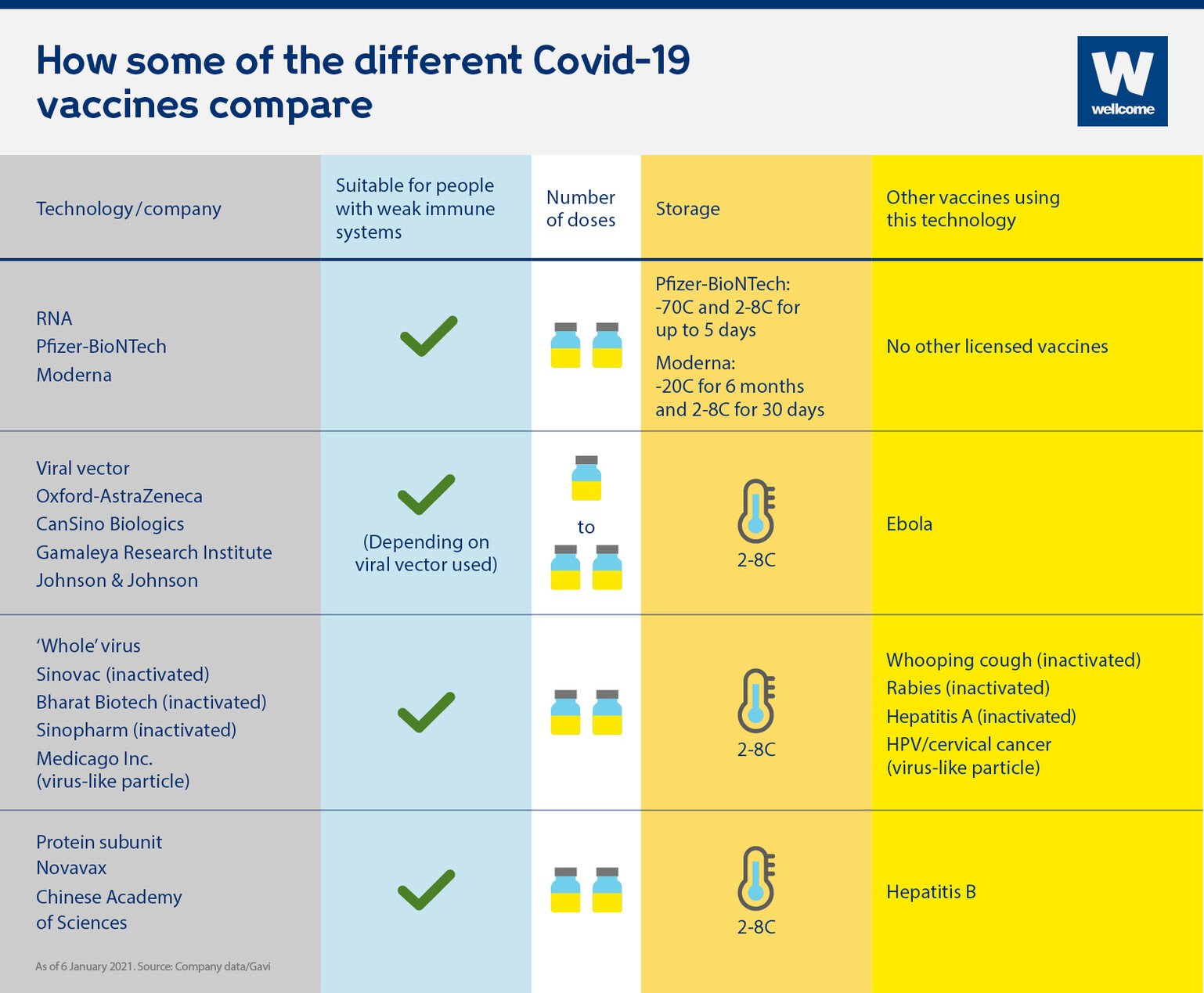
In the early days of HIV treatment and the deployment of vaccines against the H1N1 outbreak in 2009, it was seen that even when tools are available, they are not equally distributed. The challenge is to speed up and harmonize the processes to ensure that a safe product can be brought to millions of people worldwide. WHO is also coordinating global trials to assess the safety and efficacy of therapeutics for COVID-19. The WHO has pre-qualified diagnostics that are being used all over the world.
RATE OF COVID VACCINE PRODUCTION TRIAL
Immunizing the entire population of the world needs an immense amount of resources, funding, and global cooperation.Įfforts to develop a vaccine commenced in January 2020 when the WHO along with researchers from several institutions started to develop and test vaccines, standardize assays, and regulatory approaches for innovative trial designs, and define criteria for prioritizing vaccine candidates. In October, 214 countries were infected with COVID-19. However, one preventive measure that could prove to be a permanent solution to this problem is immunization. Countries are responding differently to COVID-19 by developing different strategies to address the pandemic. Globally, this rate was 2.8% in October 2020. The fatality rate of COVID-19 in India is 1.5% (1.5% of people infected with SARS-CoV-2 have a fatal outcome). Previous studies revealed how human-to-human disease transmission can cause outbreaks and how the global risk can be increased through large-scale travel and migration. Since the last 11 months, life has come to a standstill, and the vaccine is, therefore, anxiously waited. The highly contagious nature of the virus underscores the need for widespread vaccination, once vaccines are available. COVID-19 is a highly contagious disease which makes the situation very challenging for the scientific and medical communities. COVID-19 was first reported in China as an epidemic in December 2019, and the genetic sequence of the novel coronavirus was shared with the world in January 2020 for developing specific diagnostic and other health products including vaccines. India alone reported 6.69 million which was the second-highest number of reported COVID-19 cases after the United States. In early October 2020, the death counts due to the virus crossed over 1.04 million with 35.5 million cases reported worldwide. The COVID-19 outbreak had spread in over 100 countries before it was characterized as a pandemic by the World Health Organization (WHO) on March 11, 2020. This virus primarily targets epithelial cells in the respiratory and gastrointestinal tracts. COVID-19 is the name given to the disease associated with the SARS-CoV-2 virus. This new strain of coronavirus, not previously identified in humans, emerged in December 2019.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a virus in the order of Nidovirales that envelop the positive RNA strand. India and the world are at a critical juncture in the history of the pandemic where the availability of the vaccine shows a glimmer of hope-a light at the end of a dark tunnel. Important elements within the program are communications and advocacy that aim to inform the people about the vaccine and its benefits and to encourage them to get vaccinated so that the problem of vaccine hesitancy, a major deterrent, can be prevented. The state governments have developed plans for the storage and distribution of the vaccine and for the implementation of the vaccination program.

India rolled-out the COVID-19 vaccination program in January 2021. However, organizing a national vaccination program for COVID-19 is challenging because of India’s large population and fragile health infrastructure. India has also had a long history in organizing and implementing immunization programs for pregnant women and children. India is acknowledged globally to have a robust capacity for developing vaccines. Currently, five vaccine candidates are in different stages of development in India. Of the seventy vaccine candidates currently in the pipeline globally, four are available for use. The development of vaccines for COVID-19 within a ten-month period has been an extraordinary achievement given that in the past it has taken 10–15 years to develop a vaccine. The role of international organizations engaged in vaccine development, procurement, and distribution is discussed. They discuss the problems related to logistics for reaching vaccines to India’s large population.

The authors examine, in great detail, issues related to vaccine development, production, and distribution in India.


 0 kommentar(er)
0 kommentar(er)
